 |

|
 |

|
 Back to 2000 4th Quarter Table of Contents
Back to 2000 4th Quarter Table of Contents
For the purposes of this paper reference to ascorbic acid or vitamin C refers to sodium ascorbate. All in vitro studies described herein used sodium ascorbate. All intravenous vitamin C references herein refer to the use of vitamin C for injection produced by Steris Laboratories, or American Regent laboratories; both are ascorbic acid buffered to a pH range of 5.5 to 7.0 by sodium hydroxide and/or sodium bicarbonate. Background Vitamin C has potential as a chemotherapeutic agent. Rather than possessing adverse side effects as most chemotherapeutic drugs do, vitamin C has side benefits such as increasing collagen production, and enhancing immune function. We began to study the effects of vitamin C on cultured tumor cells in 1991. We found that vitamin C was preferentially toxic to tumor cells–it killed tumor cells before killing normal cells. This phenomenon first came to our attention through the work of Benade et al in 1969.1 They theorized that the preferential toxicity was due to the relative deficiency of catalase in tumor cells. This theory has since been validated by others.2 Our early findings on preferential vitamin C toxicity were published in 1994.3 In that paper we also described a so called “serum effect;” vitamin C’s toxicity was reduced by the presence of human serum. Serum’s inhibitory effects led us to the conclusion that the concentrations of vitamin C which were toxic to tumor cells in our early studies (5 to 50 mg/dL) would not necessarily be toxic in vivo. We therefore began a series of experiments in which we tried more closely to mimic the in vivo tumor microenvironment. In particular we began testing for toxicity of vitamin C toward cultured tumor cell lines using dense monolayers and hollow fiber tumor models to mimic the three dimensionality of tumors. We used human sera as culture media to include the serum inhibitory activity seen in previous assays. Using these new culture conditions we found that the cytotoxic concentration of vitamin C for most human tumor cell lines was indeed much higher than previously described. (Figure 1, p. 202). Human Vitamin C Pharmacokinetics Given the information that higher concentrations of vitamin C were required to become cytotoxic to tumor cells, we needed to learn more about the pharmacokinetics of vitamin C. There were no data on the concentrations of vitamin C that were achievable in human beings after high-dose intravenous vitamin C. We therefore began a series of experiments to yield data for modeling pharmacokinetics of high doses of vitamin C. We gave a series of vitamin C infusions to a 72 year old male who was in excellent physical condition except for slowly progressing, non-metastatic carcinoma of the prostate. Before the infusions, and at intervals thereafter, blood was drawn from a heparin lock (not the site of infusion). The plasma was separated and analyzed for plasma vitamin C concentration using a microplate-based 2,6-dichlorophenol-indophenol assay. Some of the results are given in Figure 2, (p. 202). From this experiment we observed that a 30 gram infusion was not adequate to raise plasma levels of vitamin C to a level that was toxic to tumor cells (>200 mg/dL for dense monolayers and >400 mg/dL for hollow fiber models). Infusion of 60 grams resulted in a brief (30 Figure 1. Vitamin C toxicity toward human colon cancer cells in different models. 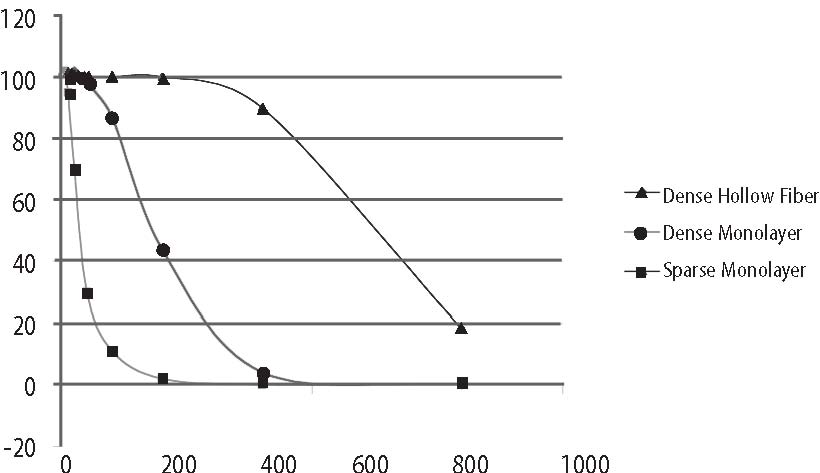 Vitamin C (mg/dL) Figure 2. Effects of 15, 30, 60, and 65 grams of ascorbate infused over various times on plasma ascorbate concentration in a 72-year-old man. 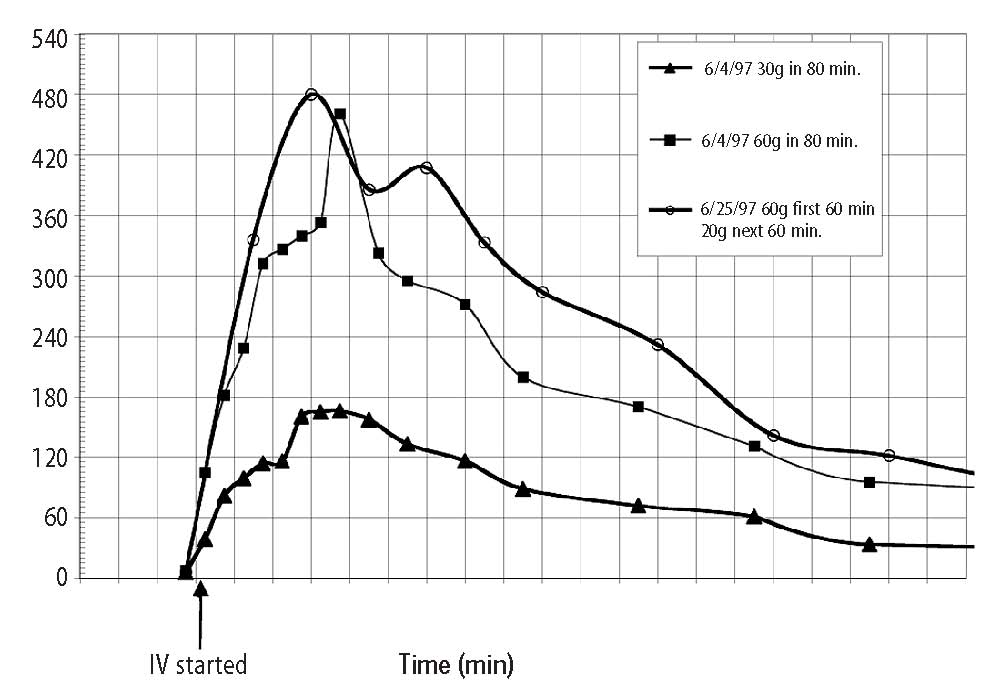 min) elevation of plasma levels of vitamin C above 400 mg/dL, while 60 grams infused over 60 minutes immediately followed by 20 grams infused over the next 60 minutes resulted in a 240 minute period in which the vitamin C plasma concentration was near or above 400 mg/dL. Ascorbate Pharmacokinetics Using data from the above experiments we designed a two-compartment model with four adjustable parameters (VP, KX, K1, K2) to fit the data. The model schematic and the data fit are shown in the accompanying Figure 3 (below). The parameter KX represents the rate of excretion of ascorbate out of the blood (renal excretion), while parameter K1 represents the rate of diffusion of ascorbate from the blood into tissue and K2 represents the diffusion rate for return of ascorbate to the blood stream from the tissue compartment. We used this two compartment model to obtain kinetic parameters from each of the in vivo vitamin C experiments where sufficient time-concentration data were obtained. We first fitted all four parameters, then fixed the blood plasma volume at 30 dL (near the average value for all experiments) and floated the three K values. Results are given in Table 1 (p.204). The average parameter values for all experiments are shown in Table 2 (p. 204) To see if there was any systematic variation in Table 2 parameter values over time, the parameter KX, and the ratio K2/ K1 for each experiment were plotted against time. The excretion constant, KX, was remarkably uniform, as was the ratio K2/K1 (Figure 4, p. 205). The tissue uptake rate constant, K1, did vary, but not in a systematic way. We use the ratio K2/K1 to point out that while K2 also varies, its variation merely compensates for variations in K1. We conclude from this analysis that the pharmacokinetic parameters of the subject did not change over a one month period of regular ascorbate infusions. Other Pharmacokinetic Studies We plotted data points from three recent studies (over a three month period) against the theoretical curve obtained using the average parameter values given above. The results are shown in (Figure 5, p. 205). Clearly, there was excellent agreement between the theoretical curve and the recent data, suggesting the ascorbate pharmacokinetics for this subject were the same over the three month treatment period. This further supports the conclusion that the ascorbate transport parameters for a given subject remain relatively constant during the time of treatment. Figure 3. Two compartment model. Injection Kx VP, CP(t) K2
Tissue Table 1. Ascorbate pharmacokinetics in vivo.
Table 2. Average parameter values.
Computer Protocol Simulations A) 1 hour at 60 g/hr A program was then constructed to B) 2 hours at 60 g/hr predict the plasma ascorbate levels in vari-C) 1 hour at 60 g/hr, then 6 hours at 10 g/hr ous protocols based on the pharmacoki-Predicted plasma ascorbate levels netic parameters obtained above. Three for these protocols are shown in Figure 6 protocols were simulated: (p.206). Prolonged infusions at higher Figure 4. Pharmacokinetic variation in parameter values over time. 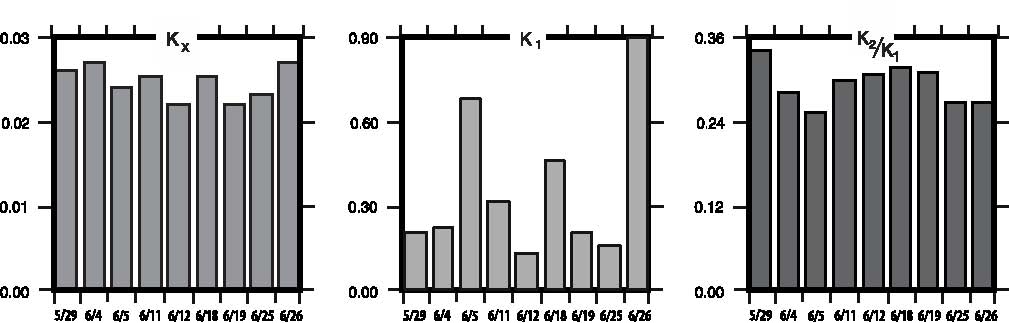 Figure 5. Plotted data points from three recent studies against the theoretical curve based on figure four values. 500 450 400 350 300 250 200 150 100 50 0 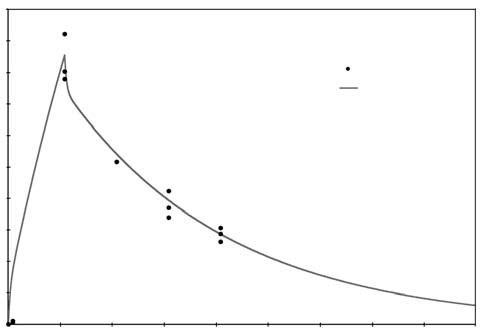 doses obviously give the highest peak values, while a “trickle” infusion can be used to maintain plasma levels at a desired level. This computer simulation can be a useful tool for examining what plasma levels we can expect from various protocols, once the pharmacokinetics for a given subject are known from an initial experiment. Effects of Human Serum Obtained from Subject Receiving Intravenous Vitamin C on Tumor Cell Growth To study more closely the probability of in vivo cytotoxic effects of intravenous vitamin C we planned and performed the following experiment. Dense monolayers of human prostate tumor cells (PC-3 from ATCC) were created in microplates. A patient with carcinoma of the prostate was given 65 grams of intravenous vitamin C (in 500 cc sterile water for injection) over 65 minutes. Serum separator blood tubes were drawn before the infusion and at intervals thereafter from a heparin lock separate from the infusion site. The final blood draw was five hours after the infusion began. Some of the collected serum was then tested for vitamin C concentration, and the rest was heat-inactivated and used as culture media for the prostate tumor cells. The cells were incubated for five days when the numbers of viable tumor cells were determined using a previously described carboxy-fluorescein diacetate/microplate fluorometer viable cell determination method. The results of the experiment are graphed in Figure 7 (p.207). Greater than 97% cytotoxicity was observed for the serum samples taken at 35, 65, and 95 minutes after the beginning of the IV vitamin C. During those periods the serum concentration was greater than 400 mg/dL. After that time the toxicity decreased. The last sample taken (vitamin C concentration 213 mg/dL) resulted in 64% inhibition compared to the controls. Potentiation of Preferential Toxicity of Vitamin C Because plasma concentrations of vitamin C of greater than 200 mg/dL are problematic to maintain, we began looking for ways to increase the sensitivity of tumor cells to vitamin C. During experimentation we found that lipoic acid (a water and lipid soluble antioxidant that recycles vitamin C) can enhance the tumor toxic effects of vitamin C. Figure 8 (p.208) illustrates dose response of tumor cells in a hollow fiber tumor model exposed to vitamin C with and without lipoic Figure 8. Effect of lipoic acid on ascorbate efficiency: SW620 human colon carcinoma cells grown as hollow fiber solid in vitro tumors exposed for 48 hours to sodium ascorbate alone or in combination with lipoic acid sodium (combo 10:1) acid. Lipoic acid decreased the dose of vitamin C required to kill 50% of the tumor cells from 700 mg/dL to 120 mg/dL. . Effects of High-Dose Vitamin C on Tumor Cell Collagen Production It is well known that vitamin C is required for the hydroxylation of proline, and that low levels of vitamin C can be a limiting factor in the production of collagen. Because many tumor cells produce collagenase and other proteolytic enzymes, we wanted to determine if vitamin C supplementation would increase collagen production by tumor cells, thereby having a balancing effect on collagenase. In an experiment, we supplemented cultured tumor cells with vitamin C concentrations that are achievable with oral supplementation (2 and 4 mg/ dL) and measured the collagen produced using a well-known method.5 We found that indeed, these concentrations of vitamin C greatly increased the production of collagen. Data are summarized in Figure 9, p.209. It is interesting to note that when we performed cytotoxicity assays of vitamin C against the PC-3 human prostate carcinoma cell lines using concentrations as high as 300 mg/dL, we were unable to detach remaining live cells from the tissue culture flasks using trypsin/EDTA. In some cases several days of treatement with high concentra-Figure 9. Effects of Vitamin C supplementation on collagen production by tumor cells. Figure 6. Theoretical blood ascorbate levels. 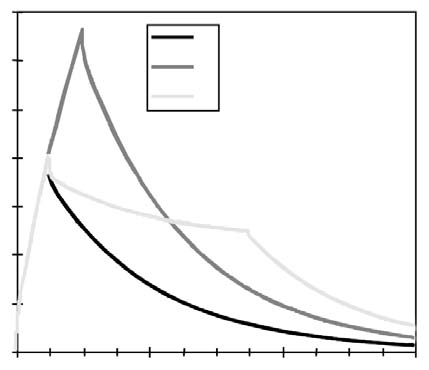 700 A 1 hour at 60 g/hr 600 B 2 hours at 60 g/hr C 1 hour at 60 g/hr 500 then 6 hours at 10 g/hr 400 300 200 100 0 0 200 400 600 Time (min) Clinical Experiences We have not observed toxic reactions to high-dose intravenous vitamin C. All patients are pre-screened for glucose-5-phosphate dehydrogenase deficiency. Patients with Renal Cell Carcinoma Treated with Intravenous Vitamin C One of us (HDR) reported positive effects of vitamin C therapy in a patient with adenocarcinoma of the kidney in 1990.4 This report described a 70-year-old white male diagnosed with adenocarcinoma of his right kidney. Shortly after right nephrectomy, he developed metastatic lesions in the liver and lung. The patient elected not to proceed with standard methods of treatment. Upon his request, he began intravenous vitamin C treatment, starting at 30 grams twice per week. Six weeks after initiation of therapy, reports indicated that the patient was feeling well, his exam was normal, and his metastases were shrinking. Fifteen months after initial therapy, the patient’s oncologist reported the patient was feeling well with absolutely no signs of progressive cancer. The patient remained cancer-free for 14 years. He died of congestive heart failure at the age of 84. 120.00 100.00 80.00 60.00 40.00 20.00 0.00 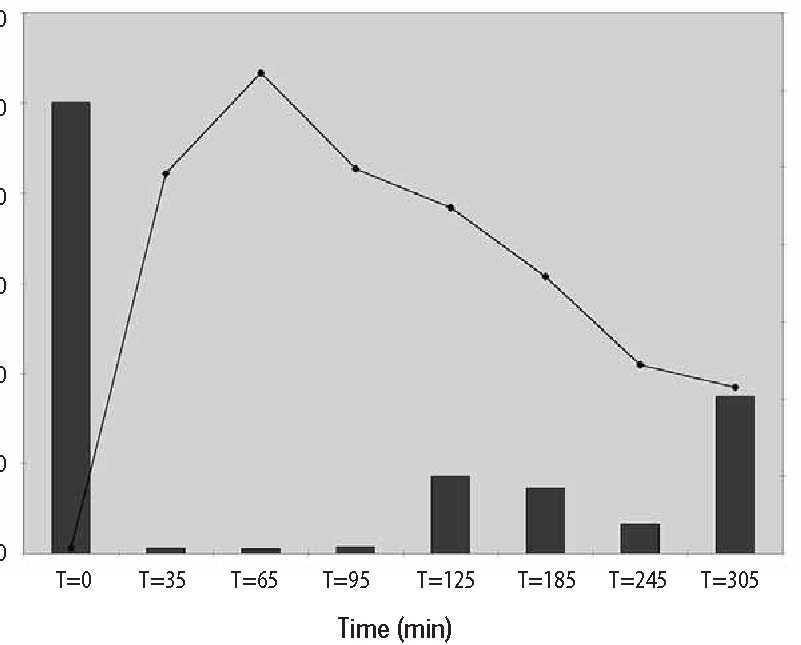 700 600 500 400 300 200 100 0
PC-3 pancreatic tumor Ovarian tumor cells 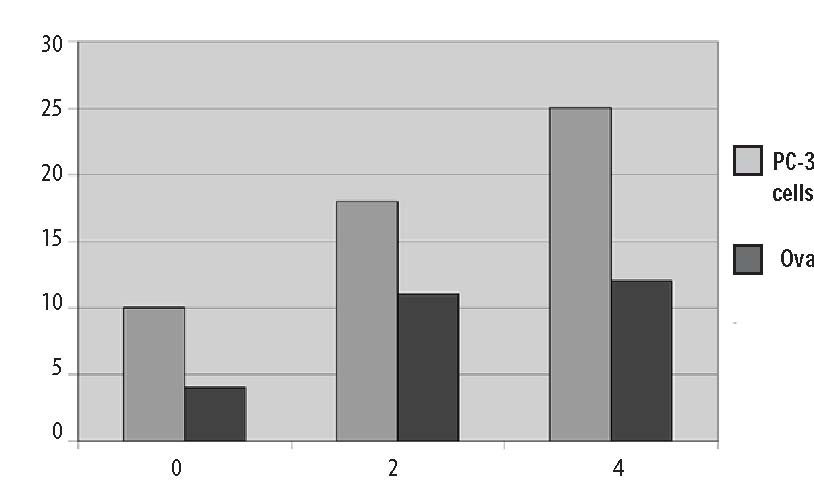 Ascorbic Acid added to Culture Media (mg/dL) A second case study, published in 1998,5 described another complete remission in a patient with metastatic renal cell carcinoma. The patient was a 52-year-old white female from Wisconsin diagnosed with non-metastatic disease in September 1995. In October 1996, eight metastatic lung lesions were found: seven in the right lung and one in the left (measuring between 1-3 cm). The patient chose not to undergo chemotherapy or radiation treatments. The patient was started on intravenous vitamin C and specific oral nutrient supplements to correct diagnosed deficiencies and a broad-spectrum oral nutritional supplement in October, 1996. The initial dose of intravenous vitamin C was 15 grams, subsequently increased to 65 grams after two weeks. The patient was given two infusions per week. Intravenous vitamin C treatments were continued until June 6, 1997. An x-ray taken at that time revealed resolution of all but one lung metastases. The patient discontinued intravenous vitamin C infusions at that time and continued taking the broad-spectrum oral nutritional supplement. A radiology report on a chest x-ray taken January 15, 1998, stated that no significant infiltrate was evident, and there was resolution of the left upper lobe lung metastasis. In February, 1999 a chest x-ray showed no lung masses, and the patient reported being well at that time. Combined Intravenous Vitamin C and Chemotherapy in a Patient with Stage IV Colorectal Carcinoma In April, 1997, a 51-year-old white male from Wichita, Kansas was first seen at our center. He was well other than having type II diabetes until the previous fall when he developed painless bright red rectal bleeding. A workup demonstrated the presence of a distal colon lesion. On December 31, 1997 he underwent an anterior colon resection and appendectomy at a local hospital. The colon tumor penetrated through the bowel wall and into the surrounding pericolonic adipose tissue. Two large hepatic metastases were discovered at the time of surgery; one was biopsied. Pathology revealed that the colon lesion was a moderately differentiated adenocarcinoma, and the liver biopsy was metastatic adeno-carcinoma. Following surgery he received chemotherapy with weekly 5-FU and leucovorin for twelve cycles with a decrease in his CEA from 90.2 to 67.7. The patient and his wife, who is an R.N., asked the chemotherapist about getting intravenous vitamin C along with the chemotherapy. The oncologist informed them that vitamin C would not be of any value. 8000 7000 6000 5000 4000 3000 2000 1000 0 12/1/96 3/11/97 6/19/97 9/27/97 1/5/98 4/15/98 7/24/98 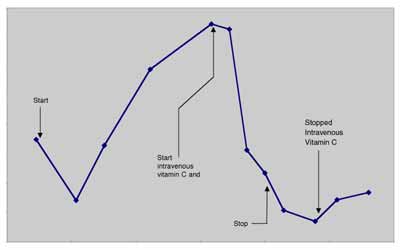 Date The patient was then seen at Pittsburg University hospital on May 13, 1997 where he underwent liver resection to segments three and five. During surgery, the stomach was mobilized off the inferior surface of the liver and a frozen section of this area was taken and confirmed metastatic adenocarcinoma. The pathology report showed metastatic carcinoma consistent with colon primary within the desmoplastic tissue and adjacent hepatic parenchyma from the stomach wall and liver. Segments three and five both contained multiple nodules. His CEA was 9.8 post surgery. The Pittsburg University oncologist informed him his prognosis was very poor and that he should go home and begin chemotherapy again. He and his wife asked this oncologist if he should use intravenous vitamin C. He responded, “I know of no studies which showed that this [vitamin C] would eradicate or delay progression of cancer.” In spite of the two noconfidence recommendations for the use of intravenous vitamin C, The patient returned to our center for infusions after recovering from surgery in June, 1997. He also began receiving weekly 5-FU (1100 mg) and Leucovorin (1300 mg) treatments administered by his local oncologist. His first vitamin C infusion was 15 grams over one hour. The dose was gradually increased during biweekly infusions. On September 9, 1997, a post-intravenous vitamin C (100 gram in 1000 cc sterile water infused over 2 hours) plasma concentration of vitamin C was 355 mg/dL. He was then started on intravenous vitamin C, 100 grams, twice weekly. His wife gave most of these infusions at home. In addition to the vitamin C, he was given recommendations for oral vitamin and mineral supplementation to increase levels of nutrients that he was found to be low in. He kept up his vitamin C infusions until February, 1998 when he traveled to Florida for a vacation. While on vacation he continued the 5-FU/Leucovorin injections. After a two weeks hiatus from the vitamin C infusions he began to experience nausea, diarrhea, stomach pain, and stomatitis; common side effects of 5-FU. The side effects stopped when he restarted intravenous vitamin C. He continued on chemotherapy and 100 gm bi-weekly intravenous vitamin C until April 1, 1998. Other than the brief period of side-effects mentioned above, he had no other side effects during the year of chemotherapy. He never experienced leukopenia, thrombocytopenia, or anemia. During April, 1998 we began to taper his intravenous vitamin C. The doses were: 75 grams, one time per week for 2 months; then 75 grams, one time every other week for 2 months; then 75 grams, on time every month for two months; and then 50 grams, one time per month for 6 months. The patient’s CEA dropped into the normal range on July 31, 1997, and has remained normal (<3.0 ng/mL) to this writing (March 20, 1999). A CT-scan in October, 1998 showed no evidence of metastatic disease. During an interview last week, he described himself as “perfectly healthy.” Comment: This report demonstrates four things about intravenous vitamin C in this patient’s case: 1) Intravenous vitamin C was not encouraged by his oncologists; 2) He did not take the advice of his oncologists on the issue of intravenous vitamin C usage; 3) His only side effects of chemotherapy occurred during a hiatus from intravenous vitamin C therapy and disappeared upon reinstatement of vitamin C infusions; and 4) For this patient intravenous vitamin C did not work against the chemotherapy, as demonstrated by his complete remission. Combined Intravenous Vitamin C and Chemotherapy in a Patient with Carcinoma of the Pancreas In October 1997, a 70-year-old white male from Southeastern Kansas was first seen at our center. After exploratory surgery in December 1997 he had been diagnosed with a low-grade mucinous carcinoma of the pancreas. During surgery there was found to be widely metastatic disease affecting all intra-abdominal organs. In January 1997 he was started on Gemzar. He had an allergic reaction to Gemzar and was placed on weekly 5-FU for 9 weeks. He was placed back on Gemzar in June, 1997 along with Decadron to counteract his allergy. In spite of chemotherapy his CA-19-9 continued to elevate until he was seen at our center. At that time his CA 19-9 was 7400 U/mL (normal <33). His first vitamin C infusion was 15 grams over one hour. His plasma concentration of vitamin C was 34 mg/dL immediately following that infusion. We expect the plasma level of a healthy person to reach 120-200 mg/dL. On his first visit he was also placed on a broad-spectrum nutritional program. The dose of intravenous vitamin C was increased to 75 gram infusions bi-weekly. His CA-19-9 serum concentration declined during this treatment until April, 1998 when he received the results of a CT-Scan of the abdomen/pelvis which showed no change compared to a CT in January. He related that he felt he was wasting his money at that time, and stopped his bi-weekly intravenous vitamin C. A graph of RB’s serial serum CA-19-9 are given in Figure 10, p.12. The evidence in this case suggests that the intravenous vitamin C was acting as a cytostatic and not a cytotoxic agent. When the patient went off the protocol, the tumors became active again. The evidence also suggest that intravenous vitamin C was working independently of the chemotherapy given the CA-19-9 level continued to decrease after chemotherapy was discontinued. This patient died at home on July 4th, 1998. Resolution of non-Hodgkin’s Lymphoma with Intravenous Vitamin C-2 Cases A 66 year old white female was diagnosed with a large perispinal (L4-5) malignant, non-Hodgkin’s lymphoma (diffuse large cleaved cell of B-cell lineage) in January, 1995. Her Oncologist recommended localized radiation therapy and Adriamycin-based chemotherapy. She began localized radiation therapy five days per week for five weeks on 1/17/95, but refused chemotherapy. On 1/13/95 she was started on intravenous vitamin C, 15 grams in 250 cc Ringer’s Lactate two times per week which she continued after completing the radiation therapy. She also began taking several oral supplements to replace those found to be deficient by laboratory testing, and empirical coen-zyme Q10, 200 mg BID. She was also successfully treated at that time for an intestinal parasite. On May 6, 1995 she returned to her oncologist with swelling and painful supraclavicular lymph nodes. One lymph node was removed and found to contain malignant lymphoma cells. In spite of recommendations for chemotherapy and more radiation, she refused and continued with her intravenous vitamin C and oral regimen. Within six weeks the supraclavicular nodes were barely noticeable. She continued intravenous vitamin C infusions until December 24, 1996. She has been followed with regular physical exams and has had no recurrence. During a telephone follow-up on 3/23/99 she was well without recurrence. Comment: This case is rare–the patient refused chemotherapy, which in all likelihood would have been curative. She also had a socalled recurrence of her lymphoma during intravenous vitamin C therapy months after her radiation therapy had ended. The possibility exists that the lymphoma cells in her lymph nodes were there at the initial diagnosis and the adenopathy occurred during immune recognition of those cells. Also of note is the fact that this patient received only 15 grams of vitamin C per infusion. According to our model, this in not a high enough dose to achieve cytotoxic concentrations of vitamin C in the blood. Therefore, any effect of vitamin C could only be attributed to its biological response modification characteristics. In Fall 1994 a 73-year-old white male farmer from Western Kansas was diagnosed with wide-spread non-Hodgkin’s lymphoma. Biopsies and CT-scan revealed bilateral tumor involvement in his anterior and posterior cervical, inguinal, axillary and mediastinal lymph node beds. Bone marrow aspirate was negative for malignant cells. He was treated with chemotherapy for 8 months that resulted in remission. In July, 1997 he began losing weight (30 lbs). He returned to his oncologist and a CT-scan at that time showed recurrence. He was placed on chemotherapy in September, 1997. In December, 1997 he developed leukopenia and then extensive left sided Herpes Zoster. As a result the chemotherapy was stopped. In March, 1998 he became a patient at our Center and began receiving intravenous vitamin C and oral nutrient supplements including lipoic acid. His vitamin C dose was escalated until he was receiving 50 grams in 500 cc sterile water two times per week. He continued on that dose for 11 months. Three months after beginning vitamin C therapy a CT-scan showed no evidence to malignancy. Another CT-scan in February, 1999 was also clear and he was declared to be in complete remission by his oncologist. Also of note is that this patient was addicted to sleeping pills when first seen at our center. After three months of intravenous vitamin C therapy he replaced the sleeping pills with Kava tea. Intravenous Vitamin C in a Patient with End-Stage Metastatic Breast Carcinoma In 1995 a hospitalized 68 year old women with widely metastatic end-stage breast cancer was seen.6 Her latest bone scan showed metastases to “nearly every bone in her skeleton.” She was experiencing bone pain which was not controlled with narcotics. At the time of her first consultation she had blood clots in both sub-clavian veins, and shortly thereafter contracted cellulitis in her left arm and hand secondary to an errant arterial blood draw. After the blood clots were treated with Activase R, she was placed on intravenous vitamin C, 30 grams per day intially, increasing to 100 grams per day over five hours. Within one week, the once bed-bound patient began walking the halls of the hospital. Several hospital staff reported that she looked like a new person. Her cellulitis cleared, and she was discharged from the hospital. At home she received 100 grams of intravenous vitamin C three times per week. Three months after starting the vitamin C therapy a bone scan revealed resolution of several skull metastases. Six months after starting the vitamin C, she fell while shopping at a mall, and subsequently died of complications from pathological fractures. Conclusion We have presented evidence that vitamin C may be useful in the treatment of cancer. In particular we have produced the following evidence: Vitamin C is toxic to tumor cells. Concentrations of vitamin C that kill tumor cells can be achieved in humans using intravenous vitamin C infusions. Infusion of a bolus of vitamin C followed by slow infusion can result in sustained concentrations of vitamin C in human plasma. Modeling of vitamin C pharmacokinetics can accurately predict plasma concentrations of vitamin C using varied infusion protocols. Lipoic acid enhances vitamin C induced tumor cell toxicity. Vitamin C in blood concentrations achievable through oral supplementation is capable of increasing collagen production by tumor cells. Vitamin C in doses up to 50 grams per day, infused slowly, are not toxic to cancer patients. Some cancer patients have had complete remissions after high-dose intravenous vitamin C infusions. Concentrations of vitamin C that kill most tumor cells are not achieved after infusion of 30 grams of vitamin C. Therefore, remissions in patients treated with this dose of vitamin C are likely to have occurred as a result of vitamin C induced biological response modification effects rather than its cytotoxic effects. References
| |||||||||

This website is managed by Riordan Clinic
A Non-profit 501(c)(3) Medical, Research and Educational Organization
3100 North Hillside Avenue, Wichita, KS 67219 USA
Phone: 316-682-3100; Fax: 316-682-5054
© (Riordan Clinic) 2004 - 2024c
Information on Orthomolecular.org is provided for educational purposes only. It is not intended as medical advice.
Consult your orthomolecular health care professional for individual guidance on specific health problems.