 |

|
 |

|
 Back to 2000 3rd Quarter Table of Contents
Back to 2000 3rd Quarter Table of Contents
Abstract Effects of a dietary supplement, d-Lenolate, on the host defence in immunocompromised mice were investigated in terms of the restoration of leukocytopenia and the protection against several microbial infections. Lenolate potentiated phagocytic activity of rat or mouse peritoneal polymorphonuclear leukocytes (PMN) in vitro or in vivo, respectively. The agent enhanced nitroblue-tetrazolium (NBT) reducing capacities of rat and guinea pig polymorphonuclear leukocytes in the peripheral blood. Oral or intraperitoneal administration of Lenolate into mice prior to X-irradiation or treatment with cyclophosphamide (CY) prevented the leukocytopenia to some extent and promoted the restoration in the phagocytic activity of both the peripheral blood leukocytes and peritoneal macrophages. In addition, IL-1 production by mouse macrophages was augmented by culturing with the agent in vitro. Oral administration of Lenolate increased significantly the survival rates of X-irradiated mice against acute systemic infections with Escherichia coli, Pseudomonas aeruginosa and Candida albicans, and intramuscular infection with Esherichia coli. The clearance of Esherichia coli from the blood of X-ray irradiated mice was also promoted by the treatment with Lenolate. These results suggest that the activation of phagocytic cell functions by Lenolate contributed to the enhancement of host defence against severe infections in patients with neutropenia induced by heavy anti-tumor chemotherapy. Key Words: d-Lenolate, host defence, leukocytopenia, microbial infections Introduction Chemotherapeutic agents or irradiation have been widely utilized in studies of anticancer therapy. As a severe side effect, however, various types of infection frequently develop in patients with defective host defence is a result of immunosuppressive treatment. Such side effects interfere with the assessment of the therapeutic efficacy of the treatment.1-5 The prevention of such side effects has become important in immunosuppressive anticancer treatments. We have studied agents with the capacity to potentiate or restore host defence mechanisms in immuno-compromised host and have found a dietary supplement, Lenolate, that has the ability to restore leukocytopenia and provide protection against microbial infections in immunocompromised mice. It was well known that phagocytes consisting mainly of polymorphonuclear leukocytes (PMN) and macrophages played an important role in non-specific host defence mechanisms.6-9 In addition, various microbial products such as Bacilles Calmette Guerin (BCG).10 Corynebacterium parvum (C. parvum),11 lipopolysaccharide (LPS),12 polysaccharide13 and phospholipids14 as well as synthetic compounds, muramyl dipeptide and its analogs15,16 have been shown to potentiate host defence against several microbial infections in experimental animal models, and to activate various functions of phagocytes.17-20 In this study, we report the restorative effect of Lenolate on defective host defence against microbial infections. The present study focused on the effect of Lenolate on enhancement or restoration of the functions of PMN and macrophages in normal or immuno-suppressed hosts. Materials and Methods Animals. Male ICR and Balb/c mice (8 weeks old), Wistar rats (220-250 g) and guinea pigs (250-280 g) were purchased from Japan Charles River Co., Ltd. (Tokyo, Japan) and used for experiments. Agents. d-Lenolate (Lenolate), a patented stereo extraction from olive leaves, supplied by East Park Research (Henderson, NV, USA) was dissolved in sterile distilled water. Lithium carbonate and prednisolone were purchased from Sigma Chemical Company (MO, USA). OK-432 (Picibanil; Chugai Pharmaceutical Co., Tokyo, Japan), a group A streptococcal preparation, was suspended in sterile distilled water to give a concentration of 25 KE/ml and used as a standard immunostimulant.21,22 Lipopolysaccharide (LPS; E. coli O55:B5, Difco Laboratories, Detroit, MI) was also used as a standard stimulant. Cyclophosphamide (CY; Endoxan, Shionogi & Co., Ltd., Osaka) was dissolved in sterile distilled water at a concentration of 20 mg/ml. Microorganisms. Escherichia coli 0111a (E. coli), Pseudomonas aeruginosa u-31 (P. aeruginosa) and Candida albicans IFO (C. albicans) were subcultured once on Tryptic Soy Agar (Nissui Pharmaceutical, Co., Ltd., Tokyo, Japan) and then cultured overnight in brain heart infusion broth (BHI; Nissui) at 37ºC. Dilutions of bacterial cultures with PBS were inoculated into mice. Treatment with immunosuppressant. Lenolate (250-500 mg/kg) was administered per os (po) and intraperitoneally (ip) into mice and 6 days later, those mice were injected ip with CY (200 mg/kg). X-ray irradiation. The irradiation was delivered by an X-ray generator (MBR-1505R; Hitachi Medico, Co., Ltd., Tokyo, Japan) operating at 150 kv, 5 mA with 0.1 mm Cu and 0.5 mm Al, 60 cm from the target focus, and each group of 10 mice was exposed to X-irradiation at 1 day before microbial infections. Infections and administration of agents. Each group of 10 X-irradiated mice was inoculated intravenously (iv) or intramuscularly (im) with the minimum lethal dose (MLD) of microbes. The mice were administered po with Lenolate (250-500 mg/kg) or OK-432 (1KE/kg) 7 consecutive days before infection. The MLD of each microorganism for X-irradiated mice was determined by repeated preliminary experiments. As a control, X-irradiated mice were injected with the same volumes of PBS via the same routes. Determination of the protective effect. The protective effect of Lenolate was determined by the survival rate of infected mice. The survival rates of mice were recorded up to 14 days after infection. Bacterial clearance test. X-irradiated mice were inoculated iv with E. coli and administered po with Lenolate immediately after infection. Two hours after infection, blood specimens were collected by puncture of the retro-orbital venous plexus. The blood samples were serially 10-fold with PBS and each dilution (0.1 ml) was spread onto plates of nutrient agar (Nissui). After agar plates were incubated for 1 day at 37 ºC, colonies were counted and colony-forming units (cfu) calculated per milliliter of blood. Counts of peripheral leukocytes. Blood specimens were collected by puncture of the retro-orbital venous plexus. Total numbers of leukocytes were counted after staining with Turk’s solution. Results were expressed as the number per milliliter of the blood. Differential counts were carried out after staining of smears with Giemsa’s solution. Preparation of phagocytes. Mice, rats and guinea pigs were inoculated ip with casein solution (1% in PBS, Sigma) of 1, 15 and 30 ml, respectively. Peritoneal exudate cells were harvested and suspended in Hanks’ solution (GIBCO, NY) (5 x 106 cells/ml) 3h later. The purity of PMN in the suspension was more than 90%. PMN in the peripheral blood were obtained by the sedimentation method with dextran23 and suspended in Hanks’ solution (5x106 cells/ml). Peritoneal macro-phages of mice were prepared as adherent cells which had been harvested 3 or 4 days after ip injection of casein solution. The peritoneal exudate cells suspended in RPMI1640 medium (GIBCO) supplemented with 10% fetal calf serum (FCS; GIBCO), 2mM L-glutamine, 100 μg/ml streptomycin and 100 u/ml penicillin were incubated at 37ºC for 1 h and nonadherent cells were removed by washing with Hanks’ solution.24 The adherent cells morphologically consisted of macrophages more than 95%. Measurement of phagocytic activity. Phagocytosis of heat-killed yeast by phagocytes was measured by a modification of the method of Miller.25 PMN or macrophages (1 x 106 cells/200 μl or culture) were mixed with yeast suspension (1 x 107 cells/100 μl or culture) and incubated at 37 ºC for 20 min in the presence or absence of autologous serum, respectively. After incubation, fuchsia solution (1% in saline) was added to the mixture and cells ingested more than one yeast cell were counted under a microscope. The number of phagocytosing cells was recorded as percentage among more than 500 cells. Phagocytic activity in vivo was measured using peritoneal exudate cells 2 h after ip injection of yeast suspension (5 x 107 cells/mouse). Measurement of Nitroblue Tetrazolium (NBT) reduction. PMN suspension (2.5 x 106 cells/500 μl) was mixed with 0.1% NBT solution (1.0 ml) and latex suspension (3 mg/ ml; 10 μl), incubated at 37ºC for 30 min and NBT-reducing cells were counted after the addition of methyl-green solution (1%) under a microscope. The NBT reducing activity of PMN in the presence of LPS (10 g/ml) was also measured.26 Assay of interleukin 1 (IL-1). IL-1 activity was determined by thymocyte co-stimulator assay described by Oppenheim et al.27,28 Supernatant from macrophages cultured with each test compound was diluted in 96 well microplates and 0.l ml of 1.5 x 106 thymocytes containing PHA (Bacto phytohemagglutinin P; Difco) was added to each well. After incubation at 37ºC for 72 h, wells were pulsed with 0.5μl [3H] thymidine ([Methyl-3H]-thymidine 5 Ci/mmol) for 5 h and harvested. IL-1 activity was expressed as the mean cpm in duplicate cultures stimulated with PHA subtracting the mean cpm in the cultures without PHA. Statistics. The statistical significance of the data was determined by the Student’s t-test. A p value of less than 0.05 was taken as significant. Results Restoration of leukocytopenia in immunocompromised mice by Lenolate. When mice were injected ip with CY, levels of peripheral blood leukocytes were markedly decreased 2-4 days after CY-treatment returning to normal levels in 8 days. In contrast, po administration of Lenolate 6 days before CY-treatment significantly prevented the decrease in the number of peripheral leukocytes Figure 1, p. 130. After X-ray irradiation, the number of peripheral leukocytes rapidly decreased from day 1 and slowly increased from day 4, but was not completely restored up to 14 days after X-irradiation. When Lenolate was administered po into X-irradiated mice before 7 days irradiation, the expected large decrease in peripheral leukocytes was not seen and the levels were restored gradually and almost completely 14 days after irradiation (Figure 2, p.130). Intraperitoneal administration of Lenolate 6 days before CY-treatment could also prevent the decrease of peripheral leukocyte levels (Table 1, p.131). Figure 1. Restorative effect of Lenolate on the decrease of peripheral blood leukocytes in mice treated with cyclophosphamide. Lenolate (500 mg/kg) was administered po into mice injected ip with cyclophosphamide (200 mg/kg) 1 day previously, and numbers of leukocytes in the blood were measured 2, 4 and 7 days after cyclophosphamide-treatment. 120 100 80 60
40 and Lenolate. 20 p < 0.05 0 days after cyclophosphamide treatment 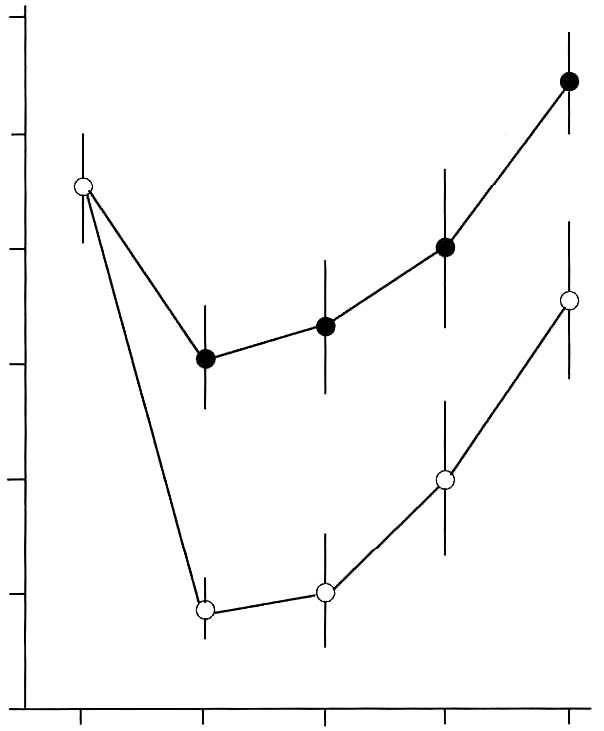 Figure 2. Restorative effect of Lenolate on the decrease of peripheral blood leukocytes in X-ray irradiated mice. Lenolate was administered po into mice 7 days before X-ray irradiation and the number of leukocytes in the blood was measured up to 14 days after X-ray irradiation. 120 100 80
40 20 0 days after X-ray treatment 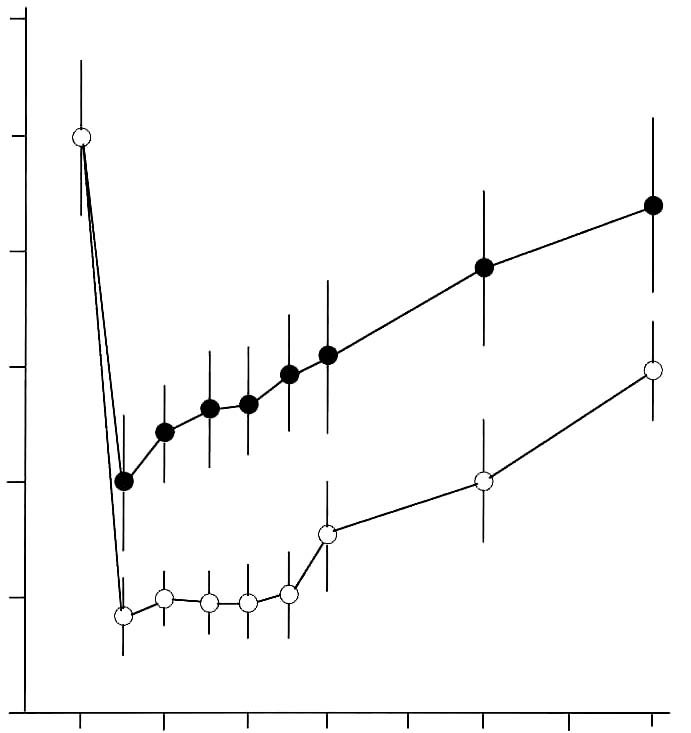 Protective effect of Lenolate against experimental infections in X-irradiated mice When X-irradiated mice were inoculated iv with E. coli (1 x 106 cfu), all of mice had died by 7 days. On the other hand, the survival rate of mice was increased by the administration of various doses of Lenolate 7 days before infection, and treatment with Lenolate (500mg/kg) resulted in complete survival of infected mice up to 14 days after infection. Treatment with OK-432 (1KE/kg) 7 days before infection increased in the survival rate approximately 60% (Figure 3A, p.132). When X-irradiated mice were inoculated im with E. coli (1 x 106 cfu), no mice survived 8 days after infection. The survival rate of infected mice increased by treatment with Lenolate dependent upon the dose administered (Figure 3B, p.132). The survival rate of OK-432 treated mice was 20% 14 days after infection (Figure 3B). Although almost all mice inoculated iv P. aeruginosa (1 x 106 cfu) 1 day after X-irradiation died by 14 days after infection, more than 80% of mice survived after the treatment with Lenolate (250 or 500 mg/kg). The survival rate of OK-432 treated mice was 60% 14 days after infection (Figure 4, p.133). After iv inoculation of C. albicans (1 x 107 cfu) into X-irradiated mice, all died by 12 days. When the mice were treated with Lenolate (500 mg/kg) for 7 days before infection, the survival rates markedly increased to over 70%. Nevertheless, the survival rate of mice reached 30% by treatment with Lenolate (250 mg/kg) or OK-432 (1KE/kg) 14 days after infection (Figure 5, p.133). Stimulation of bacterial clearance from the blood by Lenolate Following the iv inoculation of E. coli (1 x 106 cfu) in X-irradiated mice, the clearance from the blood was markedly suppressed in comparison with that in normal mice. In contrast, the clearance was enhanced or restored in mice treated with Lenolate immediately after infection. The treatment with OK-432 could also restore clearance from the blood 2 h after infection (Table 2, p. 134). Table 1. Restoration of leukocytopenia in cyclophosphamide-treated mice by Lenolate Dose Number of leukocyte/mm2† % of Agents* (mg/kg) Routes (x 102) control Control 0 92.2 ± 5.1 100.0 CY 200 ip 28.4 ± 1.3 ‡30.8 CY+Lenolate 250 ip 51.5 ± 6.8 ‡§55.9 500 ip 65.6 ± 8.8 ‡§71.1 Control 0 91.6 ± 3.4 100.0 CY 500 ip 28.8 ± 4.6 ‡71.1 CY+Lenolate 250 po 46.7 ± 2.2 ‡§51.0 500 po 61.4 ± 1.6 ‡§67.0 * Cyclophosphamide (CY) was administered into mice 1 day before and Lenolate was administered into mice every other day from 7 days before. † Number of peripheral leukocytes in the blood was determined 7 days after the treatment with Lenolate. Result was expressed as the mean ± SE. ‡ p<0.05 vs control. § p<0.05 vs CY treatment. Figure 3. Protective effect of Lenolate against experimental infections of X-ray irradiated mice with E. coli. Mice were inoculated iv (A) and im (B) with E. coli (1 x 106 cfu) 1 day after X-ray irradiation, and Lenolate or OK-432 was administered po into those mice 7 days before infections.  X-ray irradiated and infected mice mice treated with Lenolate (250 mg/kg) mice treated with Lenolate (500 mg/kg) mice treated with OK-432 (1 KE/kg). Potentiation of phagocytosis in mouse peritoneal PMN in vivo To investigate the potentiation of phagocytosis in vivo by Lenolate, yeast suspension was injected ip into mice immediately after po or ip administration with the agent and phagocytic activity of peritoneal PMN was assessed 2 h later. The treatment with Lenolate led to significant potentiations of phagocytosis to approximately 2 fold compared to those controls (Table 3, p.134). Lithium carbonate and OK-432 also increased in the phagocytosis in vivo of peritoneal PMN by ip injections (Table 3). Restoration effect of Lenolate on the suppression of phagocytic activity in peritoneal macrophages in vivo When prednisolone was administered po to mice, phagocytic activity of peritoneal macrophages was reduced to approximately 70.5% of control (Table 4, p.135). Concomitant administration of Lenolate could restore Figure 4. Protective effect of Lenolate against experimental infection of X-ray irradiated mice with P. aeruginosa. Mice were inoculated iv with P. aeruginosa (1 x 106 cfu) 1 day after X-ray irradiation and Lenolate and OK-432 was administered po to those mice 7 days before infection. 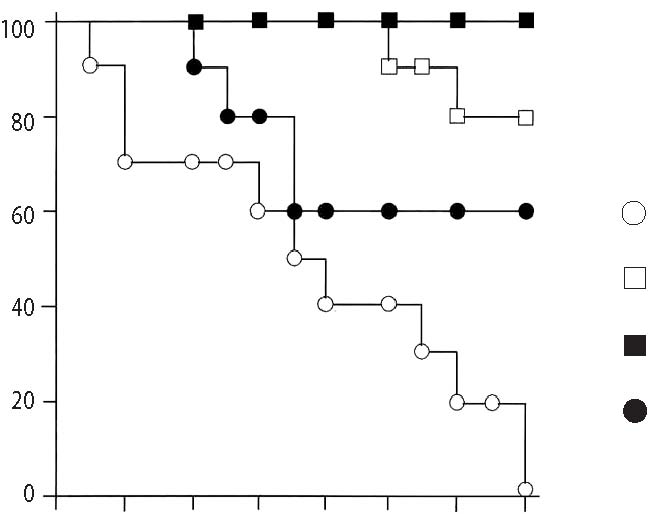 X-ray irradiated and infected mice mice treated with Lenolate (250 mg/kg) mice treated with Lenolate (500 mg/kg) mice treated with OK-432 (1 KE/kg). 02 4 6810 12 14 days after challenge Figure 5. Protective effect of Lenolate against experimental infection of X-ray irradiated mice with C. albicans. Mice were inoculated iv with C. albicans (1 x 107 cfu) 1 day after X-ray irradiation and Lenolate and OK-432 was administered po to those mice 7 days before infection. 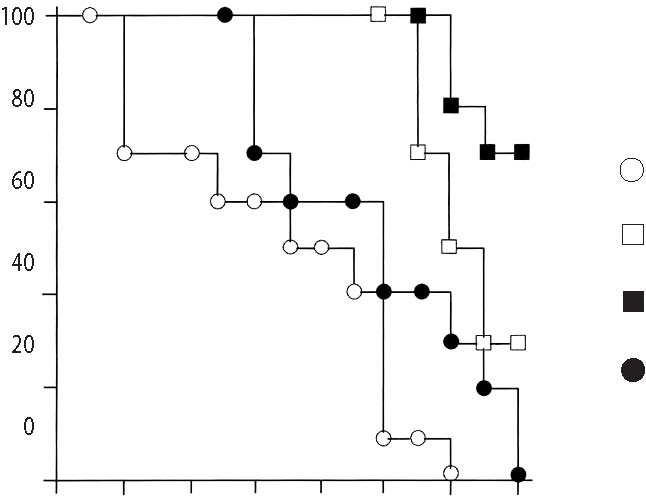 X-ray irradiated and infected mice mice treated with Lenolate (250 mg/kg) mice treated with Lenolate (500 mg/kg) mice treated with OK-432 (1 KE/kg). 02 4 6810 12 14 days after challenge Table 2. Restoration of the retardation of bacterial clearance in the blood of X-irradiated mice by Lenolate X-ray Bacterial counts† % of irradiation Agents Doses Route (x 103/ml) Control -Control52.4 ± 6.0 100.0
Mice were inoculated iv with E. coli (1 x 106 cfu) and given with Lenolate or OK-432 6 days before X-ray irradiation. †Viable bacteria in the blood were determined 2 h after the infection with E. coli. Result was expressed as the mean ±SE. ‡p<0.05 vs normal control. §p<0.05 vs X-irradiated control. Table 3. Effect of Lenolate on phagocytosis of mouse peritoneal PMN in vivo Phagocytosis† Agents Doses Routes (% of control) Lenolate0 12.4 ± 0.5 (100.0) 250 mg/kg po 20.4 ± 1.0 (164.5)§500 mg/kg po 22.8 ± 0.6 (183.9)§ Lenolate0 12.8 ± 0.4 (100.0) 250 mg/kg ip 21.4 ± 1.8 (167.2)§500 mg/kg ip 27.0 ± 2.0 (210.9)§ LiCO30 12.9 ± 2.4 (100.0) 30 mg/kg ip 15.7 ± 2.6 (121.7)§100 mg/kg ip 28.5 ± 4.0 (220.9)§ OK-4320 12.6 ± 1.2 (100.0) 1 KE/kg ip 18.4 ± 0.8 (146.0)§10 KE/kg ip 20.0 ± 1.5 (158.7)§ † Yeast suspension (5 x 107 cells/mouse) was injected ip into mice immediately after treatment with eachagent and peritoneal fluids were harvested 2 h later. Results were expressed as the mean±SE.§ p<0.05 significant vs control. Table 4. Effect of Lenolate on the suppression of phagocytic activity in mouse peritoneal macrophages in vivo by prednisolone Doses Phagocytosis† Agents (mg/kg) Route (% of control) Control 0 15.6 ± 2.4 (100.0)§Prednisolone 30 po 11.0 ± 1.4 (70.5)
†Lenolate was administered po to mice immediately after the treatment with prednisolone and once a day forconsecutive 7 days. Casein solution (1%) was inoculated ip into the mice 4 h after the treatment withprednisolone and peritoneal macrophages were prepared 3 days after the treatment. Yeast suspension (1 x 10cells/culture) and further incubated at 37ºC for 30 min, and then the phagocytic activity was measured.Results were expressed as the mean ±SE.§p<0.05 significant vs prednisolone treatment. Table 5. Effect of Lenolate on NBT reduction by rat and guinea pig peripheral PMN in vivo NBT reduction† Animals Agents Doses Route (% of control) RatControl 0 24.0 ± 0.8 (100.0) Lenolate 500 mg/kg po 45.2 ± 2.4 (188.3)§OK-432 1 KE/kg po 34.2 ± 1.0 (142.5)§ Guinea pig Control 0 14.6 ± 0.8 (100.0) Lenolate 500 mg/kg po 28.1 ± 1.8 (192.5)§250 mg/kg ip 19.4 ± 1.6 (132.9)§500 mg/kg ip 41.5 ± 2.3 (284.2)§ † Peritoneal PMN were prepared by dextran sedimentation method 2 h after administration with each agent and NBT reduction was measured in vitro. Results were expressed as the mean±SE. § p<0.05 significant vs control. from the suppressed activity to normal level at doses of 250 and 500 mg/kg (Table 4). Potentiation of NBT reduction in peritoneal PMN of rat or guinea pig in vivo Peritoneal PMN were obtained from rats or guinea pigs 2 h after administration of Lenolate and the NBT reducing capacities was compared with that of normal PMN. Treatments of Lenolate resulted in the marked potentiation of the NBT reducing capacity at different routes of administration (Table 5, above). Potentiation of IL-1 production in murine peritoneal macrophage cultures Peritoneal macrophages were obtained from mice after administrations of the agent. After peritoneal macrophages were cultured for 2 h, harvested supernatants were used as sources of IL-1. When macrophages were administered with Lenolate (500 mg/kg), the IL-1 production was increased to about 2 fold in comparison with control mice (Figure 6, below). OK-432 and LPS also enhanced IL-1 production of macrophages (Figure 6). Discussion The results of this study indicate that treatment of immunocompromised mice with Lenolate enhanced the restoration of leukocyte levels to normal and provided protection against experimental infections with E. coli, P. aeruginosa and C. albicans. It appeared that PMN were the primary effector cells in the elimination of a variety of bacteria from tissues and blood. The significance of PMN in the protection against microbial infections was made clear from experiments on immunologically compromised X-irradiated and CY-treated hosts.8, 29-31 In the early phase of infection with E. coli or P. aeruginosa, protection of mice has been attributed mainly to PMN.6,9 It was also shown that PMN contributed to protection against infection with C. albicans.7,32,33 Furthermore, the connection between decreased number of PMN and risk from infections has been clearly shown in human therapy.34,35 In the present study, treatment with Lenolate enhanced the clearance of E. coli from the blood of immuno-compromised hosts. In addition, our present study has indicated that number of PMN increased primarily along with the restorative effect of Lenolate on the leukocytopenia in immuno-compromised mice. Therefore, those observations strongly suggest that the rapid restoration in the number of peripheral PMN by Lenolate contributed to the enhancement of host defence in immuno-compromised mice. In fact, this interpretation is supported by several studies indicating that lithium carbonate, which induces a reversible leukocytosis, might be capable of ameliorating the leukocytopenia associated with systemic chemotherapy.36-38 Figure 6. IL-1 production of mouse peritoneal macrophages activated by Lenolate in vivo. Peritoneal macrophages of BALB/c mice (5 x 106 cells/ml) were cultured with test compound at 37ºC for 24 h and IL-1 activity in the culture supernatant was determined by thymocyte co-stimulator assay. Results were expressed as the mean cpm in the culture (duplicate) stimulated with PHA subtracting the mean cpm in the culture without PHA (Δcpm). 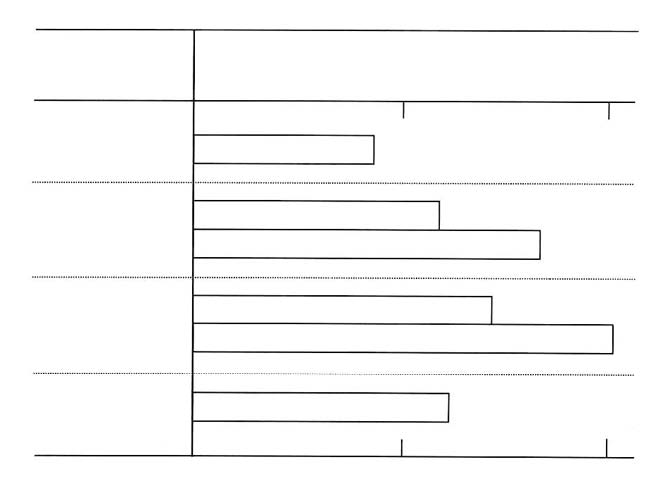 In this study, present data indicated that Lenolate could activate both phagocytic and NBT reducing capacities of PMN or macrophages in vivo. It was also shown that NBT reducing activity of leukocytes represented processes of the production of activated oxygens and closely related to the bactericidal steps.26,39-42 Therefore, it appears that the activation of phagocytic cell functions by Lenolate also contributes to protection against various microbial infections in immunocompromised hosts. Cohen et al.43 reported that PMN from psychiatric patients who were given lithium carbonate exhibited normal functions in chemotaxis, random mobility, phagocytosis and bactericidal activities. In this study, phagocytic function of PMN from mice received lithium carbonate was potentiated by treatment with high doses (Table 3, p. 134). However, the activation of phagocytosis by Lenolate was seen in mice treated even with low doses (Table 3). These findings strongly suggest that the agent may be effective on the clinical use as well as lithium carbonate. It was well known that IL-1 had a variety of important immunological functions including the augmentation of mitogenesis of thymocyte or T cell activation to interleukin 2 (IL-2) production, and B cell proliferation to antibody formation.27, 28 As the production of IL-1 in murine macrophages was augmented by the administration of Lenolate (Figure 6, p. 136), the agent appears to act on the potentiation of specific immune responses to antigens or pathogens. References 1. Devita, VTJr, Serpick AA, Carbone, PP: Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Int Med, 1970; 73: 881-895.
causes of past therapeutic failure. Lancet II, 1976; 289-291.
USA, 1978; 75: 3395-3399.
6: 587-592. 809-810.
D: The use of lithium carbonate to reduce infection and leukopenia during systemic chemotherapy. New Engl J Med, 1980; 302: 257-259. |

This website is managed by Riordan Clinic
A Non-profit 501(c)(3) Medical, Research and Educational Organization
3100 North Hillside Avenue, Wichita, KS 67219 USA
Phone: 316-682-3100; Fax: 316-682-5054
© (Riordan Clinic) 2004 - 2024c
Information on Orthomolecular.org is provided for educational purposes only. It is not intended as medical advice.
Consult your orthomolecular health care professional for individual guidance on specific health problems.