 |

|
 |

|
 Back to 1998 2nd Quarter Table of Contents
Back to 1998 2nd Quarter Table of Contents
Abstract A clinical study of 100 patients reveals that persons who eat large quantities of dietary protein (more than 30g/day) generate high levels of acid which must be neutralized before being eliminated from the body. This paper reports the findings of the clinical case study on the effect of acid versus alkaline residue on the body’s homeostasis, and the theory that human daily protein consumption is excessive and conducive to ill health. The impact of ammonia on urine pH is also critical factor in this examination, as are the transformation of urine from acid to alkaline, and differences between alkaline urine of a vegetarian and alkaline urine of a heavy protein eater. Keywords: protein, ammonia, alkaline urine, acid urine, bladder irritation. Introduction The fact that individuals need to eat less dietary protein is not new. Initial scientific studies undertaken more than 20 years ago1 found that consuming excess dietary protein created an acid environment in the human body.2 Since the human body was designed to function, for the most part, in an alkaline environment,3 it is reasonable to assume that an over-abundance of acid in the body leads to disease and ill-health. Earlier scientific exploration into the harmful effects of excess dietary protein on the human body has been overshadowed by the more popular and promoted belief that fat–not protein–is the major dietary offender.4 in recent years, scientific examinations have focused on the relationship of fat to disease, rather than the relationship of protein to disease; therefore, current literature on the disease-producing effects of excess dietary protein is inconsistent and hard to find. The literature that does exist was used to support current clinical findings and to re-establish the initial claim that excess protein consumption creates acidosis. Methods and Materials Data from this clinic show the deleterious effects on pH levels in bodies of patients ingesting excess dietary protein. In conducting 100 pre-employment clinical examinations for a local industry, a urinalysis was performed on each subject as part of the routine physical. Urine color, pH, specific gravity and protein, as well as glucose, ketone and blood levels in the urine were analyzed. Investigations into the amount of protein necessary to sustain bodily function at optimal levels vary widely in past research. McDougal,5 Robinson,6 and Gebhardt and Matthews,7 conducted extensive and independent investigations on dietary protein consumption and presented diametrically opposed analyses of acceptable levels that ranged from as low as 30g/day (McDougal)) to as high as 150g/day (Gebhardt and Matthews). In a more recent recommendation, the U.S.A. Select Department of Health and Human Resources offered other, more contrasting, daily dietary protein intake guidelines.8 McDougal found that excess consumption of dietary protein at levels greater than 30g/day generates high levels of acid that must be neutralized before being eliminated from the body. It is, however, the Schuette et al. analysis that is revelatory.9 The study by this group reported a substantial decrease in calcium retention and an increase in urinary calcium among groups of subjects when protein in diets increased from 47g/day to 112g/day. In this study, two of the nine subjects whose protein intake was increased to 47g/day, immediately showed a negative calcium balance. They reported that increasing protein content in the diet also increased urinary calcium loss. Additionally, in the same subjects, it was shown that increasing the intake of fruits and vegetables by 50% had no beneficial effect on calcium retention. Also supporting this evaluation is a study by Ansari, Canterbury and Bast which recommends moderate consumption of high acid ash foods (meat, poultry, fish, eggs and cereals) in order to decrease calcium excretion and bone loss.10 These studies show that when particular physiological systems within the body are exceeded, depletion of calcium reserves becomes immediate and inevitable. Past estimates of human adult protein needs showed that as little as 2.5% of daily caloric intake could be safely provided in the form of protein, an amount equivalent to a little less than 20g for an adult male.11 in fact, while not recommended, it has been proven that humans can survive for long periods of time in excellent health by satisfying their body’s entire nutritional needs with potatoes and water alone.12 Results The urinalyses of the 100 patients involved in this clinic’s pre-employment screening study showed 82 subjects with a urine pH below 6.0 and no symptoms of burning on urination. Symptoms of burning on urination and urinary pH levels of 7.0 or above were present in 10 subjects, while the remaining eight subjects had no symptoms of burning on urination but also showed urinary pH levels of 7.0 or above. Determining the difference between subjects showing a urinary pH above 7.0 with no presentation of symptoms of burning on urination became a focus of literary examination, and led the researcher to hypothesize that (1) either another constituent not associated with pH was responsible for the burning; or, (2) another ingredient was responsible for the alkaline pH, and that ingredient was responsible for the alkaline pH and the burning. Subsequent followup research with these subjects over the next year, including periodic urinary pH testing, and monitoring of daily dietary protein intake by calculating the number of protein grams consumed per meal (i.e. 3 oz. beef sirloin=20g/protein), convinced the researcher that excess dietary protein was the contributing link to acidosis. Results from the clinical tests indicate that on a high protein diet very acid urine is produced initially, but if high protein intake continues, a very alkaline urine occurs as a result of ammonia formation. Excess acids in the body are neutralized by sodium from the alkaline reserve. When these reserves have been utilized for an extended period, the sodium level is reduced. When this transpires, the body excretes ammonia (NH3) directly into the urine, giving the urine an alkaline pH. Additionally, it has been observed that when excessive amounts of protein are consumed (more than 30g/day), more sodium is withdrawn and eliminated in the urine which forces the body to turn to calcium, the next best alkalizing mineral, to aid in the neutralization process.13 In other related investigations, the results of a longterm study by Allen and Margen revealed that increasing nitrogen (N) grams in the diet of subjects to 36g/ day accompanied a urinary calcium increase from an average of 191mg/day on 12g N, to 277mg/day on 36g N.14 Allen/ Margen reported that all subjects in their study were in negative calcium balance on the high protein diet, with an average negative balance of 137mg/day. This rate of calcium loss, presumably largely skeletal, would amount to 50g calcium, or approximately 4% of total skeletal calcium per year. This study also concluded that implementation of a high calcium diet is unlikely to prevent negative calcium balance and probable bone loss induced by consistent ingestion of high levels of protein. A similar study into the effect of protein from meat on the body’s metabolism by Sanchez et al compared bone mineral mass between lacto-ovo vegetarians and omnivorous women over a period of 15 years after menopause and concluded that omnivorous women lost 35% of bone mass as compared to lacto-ovovegetarians who had lost only 18% of bone mass.15 In addition, Wachman and Bernstein concluded that the calciuric effects of high protein diets are secondary to the acid produced during their metabolism. These acids were held to be constantly buffered by bone, and the increased incidence of osteoporosis with age represents, in part, the result of a lifelong utilization of the buffering capacity of the basic salts of bone for the constant assault against pH homeostasis.16 Discussion The idea that protein may be responsible for increasing urinary ammonia and acidosis is gaining in its base of support. For instance, while performing amino acid urinary analyses to ascertain why some spinal cord patients at Walker institute in Pacific Palisades were losing significant amounts of muscle mass, Dr. Judith Walker unexpectedly found “huge increases” in urine ammonia levels.17 Whereas the normal urinary ammonia level ranges from 0-50 mg/mL, some of Dr. Walker’s patients showed levels between 16,000 -20,000 mg/ml. Following the initial urinalyses, Dr. Walker monitored the pH levels of those same patients over several months and found the majority of those patients presented consistently with pH levels of 6.0. In cases where the pH levels were higher, it was determined the increase was due to the accumulation of ammonia. Consider also a study on urinary acid excretion (Figures 1 and 2, p. 92) by Lutz and Linkswiler which found that within the first 24 hours after increasing dietary protein intake (from 50g/day to 110g/day), the mean urinary pH of their subjects had decreased by 0.5 units, and 12 days after the change by more than one unit, but began to increase, once again, by day 30.18 In the pre-employment screening study of patients in this clinic it was observed that (Figure 3, p. 93) symptoms of burning on urination accompanied by a high pH began in patients around day 30, and increased steadily for approximately another 30 days, eventually yielding an alkaline urine. In studying the formation of uric acid, consideration must also be given to the process by which alkaline ash is produced in a vegetarian, versus the acid ash produced by a meat eater.19 Vegetables, even acid fruits, usually have an alkaline effect and produce an alkaline urine because they contain more calcium, magnesium, sodium, and potassium; whereas, meats, fish, grains, and eggs are said to have an acidifying effect on the body as they leave an acid ash of nitrogen, phosphorus, chloride, and sulfur. In a study on the acute effect of milk proteins on urinary acid, it was demonstrated that milk protein severely decreases serum uric acid, while soy protein increases it. In both circumstances, however, elimination of uric acid is increased, and the chain of events process that takes place in eliminating excess protein (Figure 4, p. 93) automatically proceeds to the next physiological step while maintaining maximum utilization of all previous processes, yielding an overall metabolic acidosis and, ultimately, producing an alkaline urine.20 Conclusions This clinic’s analysis of urinary pH levels among its study participants, coupled Figure 1. Dietary protein and calcium metabolism before and after increasing dietary protein 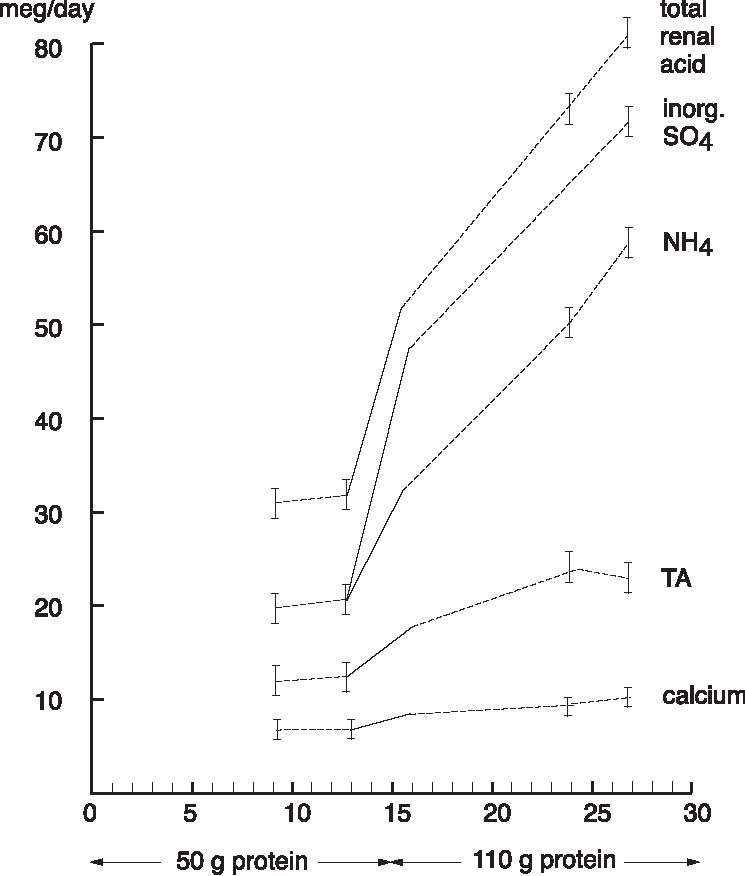 Figure 2. Urinary pH before and after increases in dietary protein 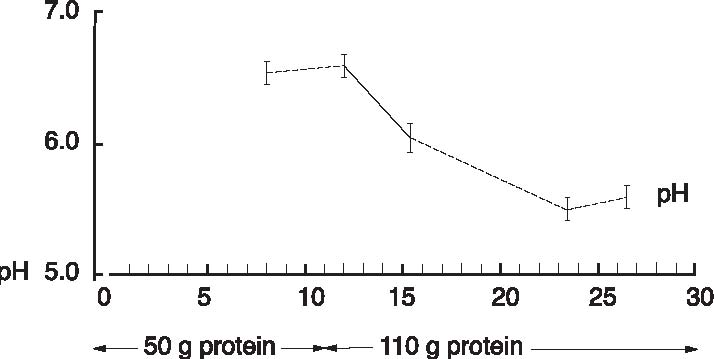 The Body’s Negative Response to Excess Dietary Protein Consumption Figure 3. Pre-employment screening. On day one 18 patients presented with urinary pH levels at or above 7.0. The pH levels continue to rise in all patients through day 30, regardless of whether or not they exhibited symptoms of burning on urination. 19 had burning on urination; 8 had no burning on urination; 10 were meat eaters; 8 were vegetarians 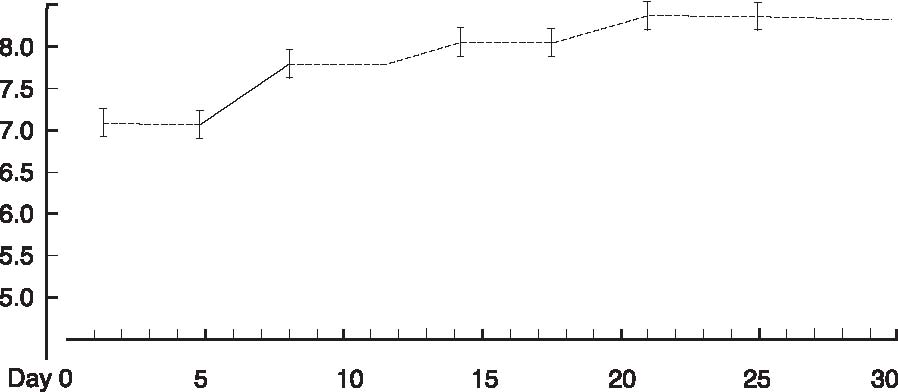 Figure 4. Chain of events caused by excess protein. This chain of events not only illustrates how the alkaline reserve is depleted but also why a high protein consuming patient can register an alkaline urine pH although his entire system is acidotic. Sodium taken from alkaline reserve produces acid salts to be eliminated. Acid urine Nitrogen eliminated naturally as urea. Acid urine Nitrogen eliminated as uric acid. Acid urine Alkaline reserve depleted. Acid urine Nitrogen eliminated directly as Acid urine Nitrogen eliminated directly as Alkaline ammonia in urine. Alkaline urine Bicarbonate and ammonia eliminated directly in urine Alkaline urine Calcium starts being used as secondary buffer Alkaline urine Protein, ammonia, and bicarbonate in urine Alkaline urine with the findings represented in the aforementioned protein studies suggest: (1) excess consumption of animal protein (over 30g/day) causes acidosis and stress to the body; and (2) the average American adult consumes more protein on a daily basis than can safely and effectively be utilized by the body. This clinic promotes that patients should ideally consume no more than 20g/protein/day with 30g/protein/day being the maximum acceptable level of dietary protein consumption. When excess protein causes formation of strong acids that must be neutralized and eliminated by the body, this process requires a plentiful supply of sodium which, if used, can comprise a large portion of the alkaline reserve. The alkaline reserve is best maintained by a diet consisting predominantly of fruits and vegetables, and if excessive amounts of protein are consumed, more sodium is withdrawn than is replaced, eventually depleting the sodium reserve. The body then turns to the next best alkalizing mineral, calcium, which is available in bones. All of the buffer systems are affected, and as the levels of acid and protein rise, cellular congestion occurs, the entire body begins to slow down and chronic degenerative disease could soon follow. On a high protein diet the body progresses through cycles of more protein, more acid, less sodium, and inevitably disease, while ultimately creating an alkaline urine as a result of ammonia formation. Although it appears desirable to maintain an alkaline urine, the alkaline environment must be supplied by a strong vegetable/ fruit diet, rather than ammonia created from excess meat ingestion and depletion of the body’s calcium reserves. References 1. Mazess RB, Mather W: Bone mineral content of north alaskan eskimo. Am J Clin Nutr, 1974; 27: 916. 2. Morter Jr, MT: Your health your choice, your complete guide to wellness, nutrition and disease prevention. Hollywood, FL: Fell Publishers, 1990; 26.
|

This website is managed by Riordan Clinic
A Non-profit 501(c)(3) Medical, Research and Educational Organization
3100 North Hillside Avenue, Wichita, KS 67219 USA
Phone: 316-682-3100; Fax: 316-682-5054
© (Riordan Clinic) 2004 - 2024c
Information on Orthomolecular.org is provided for educational purposes only. It is not intended as medical advice.
Consult your orthomolecular health care professional for individual guidance on specific health problems.